Why Choose Sonosite: Durability
SoundCaring
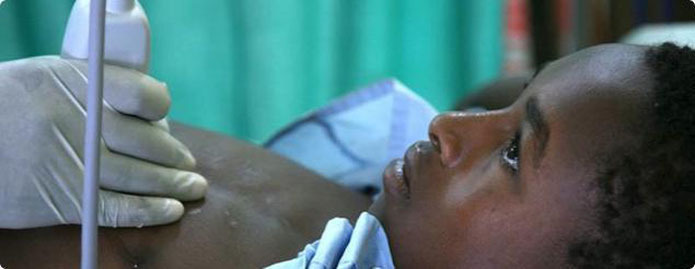
Sonosite SoundCaring and Global Health Humanitarian Programs
For expectant mothers in a rural village, an ultrasound diagnosis can mean the difference between a safe and hazardous delivery. For children suffering from rheumatic heart disease in sub-Saharan Africa or South Asia, an ultrasound diagnosis can lead to life-saving treatment. Because our equipment is uniquely suited for these austere environments, we feel compelled to bring this valuable technology to those who need it most. We continue to be humbled and thrilled by the stories recipients tell us of how our systems are impacting their communities.
Our SoundCaring and other Global Health humanitarian programs make Sonosite hand-carried ultrasound systems available to those working in remote, low-income communities with little or no access to necessary medical equipment.
Sonosite SoundCaring Application
Interested organizations must meet certain eligibility requirements as outlined on the application. Applications should be submitted at last three months prior to the requested delivery date. Download application.
Careers
Sonosite — Visualize Your Future
Match your sense of purpose with your career choice. From high-resource environments such as research facilities, hospitals, and clinics to the most remote and austere settings, clinicians around the world rely on Sonosite ultrasound systems.
Sonosite was founded on a simple premise: ultrasound systems should be able to go anywhere. We proved it. Sonosite ultrasound systems have been used on Mount Everest, for disaster relief, on the battlefield, in aircraft, and on ships. And our innovation keeps on going, not only at Sonosite but also at VisualSonics, our pre-clinical subsidiary, the world-leader in real-time, in vivo, high-resolution micro imaging systems.
Grow Your Career
As a dynamic and fast-growing global company, we offer challenging opportunities so you can grow your career. As an organization, Sonosite
- Values and embraces cultural diversity
- Supports your development needs through a stringent performance management process that can support your performance and growth
- Offers numerous training opportunities, including online options; learn anywhere, anytime
- Provides benefits programs competitive in each market
Help Advance Healthcare and Research
About
FUJIFILM Sonosite, Inc., the world leader in bedside and point-of-care ultrasound, delivers solutions that meet imaging needs of the medical community. With its acquisition of VisualSonics’ ultra high-frequency micro imaging technology, Sonosite continues to influence the future of medical ultrasound in both the clinical and preclinical markets.
A Brief Company History
From behind-the-scenes experimental work for the U.S. Department of Defense to today’s highly advanced ultrasound systems used around the globe, Sonosite has been defining and redefining next-generation point-of-care (POC) ultrasound as its recognized market leader. Since the company’s early pioneering days in the 1980s, Sonosite has continued to enjoy remarkable growth while earning worldwide recognition for its progressive product line, educational programs, and advocacy for a broader understanding of ultrasound’s multiple benefits.
Sonosite's Global Health Program
Portable Ultrasound Machines for Medical Missions
According to the World Health Organization, over 60% of the world’s population has no access to ultrasound machines for medical diagnosis. We're working to lower that statistic.
About FUJIFILM VisualSonics
About FUJIFILM VisualSonics
FUJIFILM VisualSonics, a wholly owned subsidiary of FUJIFILM Sonosite, designs and manufactures Ultra High Frequency ultrasound imaging systems, for both research and clinical use.
FUJIFILM VisualSonics specifically focuses on Ultra High Frequency (UHF) ultrasound machines. For the first 3cm of a scanned body, UHF ultrasound provides images at much higher resolutions—as fine as 30μm—than other imaging systems currently available.
Quality Assurance
Quality Assurance | Regulatory Affairs |
Regulatory Documents
US/CanadaEuropean UnionAll Other CountriesSafety/EMC
Sonosite is comprised of an innovative team of professionals with many years of experience in medical diagnostic ultrasound technology. Sonosite is at the forefront of defining a world-class standard of quality management for ultrasound manufacturing. Sonosite, Inc. is an FDA registered firm as a specification developer and manufacturer of medical devices.
Sonosite products are designed, manufactured, and distributed in full accordance with the requirements of FDA’s Quality System Regulation. We are regularly inspected by BSI - the British Standards Institute, which is Sonosite’s European “notified body” under the medical device directive. Regulatory documents for both individual devices and Sonosite’s quality management system are available.
United States/Canada
The 510(k) pre-market notification process is the most common method used in the United States for describing the design and manufacture of medical devices which the US Food and Drug Administration (FDA) uses to determine a "substantial equivalence" decision to authorize marketing of a medical device within the United States.

